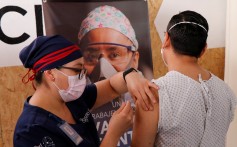
Britain became the first country to approve a drug developed by Pfizer and BioNTech, with regulators in the US and Europe also considering applications
- Chinese drug firms have yet to submit final data even though a million people have already received emergency shots
Britain approved the emergency use of a Covid-19 vaccine developed by Pfizer and BioNTech on Wednesday, becoming the first Western country to begin vaccinating its population.
Other Western countries may soon start the process of mass innoculation if this and other vaccines, including one made by Moderna and another produced by Oxford University and AstraZeneca, gain approval from regulators in Europe and the United States.
In the United States, the Food and Drug Administration will hold a meeting on December 10 to discuss the emergency use authorisation for the Pfizer-BioNTech vaccine, while Moderna has also applied for FDA approval.
Interim data show that the two vaccines have an efficacy of around 95 per cent, although questions remain about how long the immunity will last.
The European Medicines Agency will meet the following day to discuss the criteria for emergency use, and Pfizer and BioNTech have also applied for conditional marketing approval.
The Oxford/AstraZeneca drug is also being assessed by the British regulator. However, there are questions about the way the data was presented and why participants were given different doses, which may cause problems with the FDA.
But while people in Europe and the United States can expect the vaccines to become available soon, Chinese drug companies have yet to present their trial data even though the country was among those leading the way in the early phases of the research process.
So has it lost the vaccine race yet?
Huang Yanzhong, a senior fellow for global health at the Council on Foreign Relations in the US, said Western vaccines might have gathered efficacy data more quickly because of the widespread transmission of the coronavirus in the US and Europe.In phase three trials, scientists compare the infections between volunteers who receive a placebo and a vaccine. The more infections, the more quickly a trial can generate enough data.
“The biggest challenge for China is that some of the countries that work with China’s vaccines turned out to have fewer cases [than expected], that is sort of ironic,” he said, citing the United Arab Emirates, where large-scale trials of a vaccine developed by Sinopharm were held.
On November 11, just a day after Pfizer-BioNTech published data showing their vaccine had a high efficacy rate, state-owned Sinopharm released a statement saying that its clinical trials would finish soon with “better-than-expected” data, although it did not elaborate.
Last week it was announced that trials for another Chinese vaccine, made by Sinovac, in Brazil had collected enough data and an announcement will be made this week.
These findings could prove crucial to China’s vaccine diplomacy, including its efforts to expand its influence in Southeast Asia.
Last month one of the key targets of the initiative, Indonesia, said it would not grant emergency use authorisation to Sinopharm’s product this year because the data from Brazil was not available.
Huang said the data released by other researchers had put pressure on China as it had promised to supply vaccines to other countries, and they are still waiting for data.
“We cannot deny there is a vaccine race going on … when China kicked off their vaccine development projects, they did not hide their ambition that they wanted to be number one in successfully developing the vaccine,” he said.
“They view that as an issue of national pride and to showcase China’s capability in high technology areas.”
Just days after Pfizer and Moderna released their results, Chinese state media reported that Sinopharm had started the process of applying for conditional approval for two vaccines.
Chinese regulators also approved the emergency use of Sinopharm and Sinovac products months before the final clinical trials had concluded, and more than a million people have already been given the vaccine.
Medical specialists said the products developed in Europe and the US are likely to get approval from the World Health Organization before the Chinese ones.
The regulators in those jurisdictions, along with Japan, are on the WHO’s list of stringent regulatory authorities – which makes them eligible to be fast-tracked for approval – but China is not on the list.
“If it is coming from a country where the national regulatory authority is not considered stringent, then there may be additional steps or additional questions … That means there can be a little bit of delay in review ,” Jerome Kim, director-general of the International Vaccine Institute, said.
Chinese officials and drug firms have repeatedly said there have been no infections among the million-plus people given the emergency use vaccine. Around half of them have travelled abroad after being given the injections, which was cited as proof they could protect people.
In early November, Sinopharm chairman Liu Jingzhen said 81 out of the 99 employees in Huawei’s Mexico office had been given the vaccine. Ten of the unvaccinated workers later developed Covid-19, but none of those who received the injections became infected.
But Kim pointed out: “They were vaccinated because they were going overseas, that is not part of a trial and that is an anecdotal report, that is not part of the trial. That is not efficacy.”
Peter Smith, a professor of tropical epidemiology at the London School of Hygiene and Tropical Medicine and an adviser to the WHO, said the global health body would want to see proper data from the final clinical trials.
“I think it is unlikely that WHO will issue an EUL [emergency use licence] without seeing convincing efficacy and safety data,” he said.
But even if the approval process takes longer for Chinese vaccines, as long they are largely comparable with their Western counterparts, the vaccines will be snapped up quickly by developing nations that cannot get hold of Western vaccines.
“The notion of competition is irrelevant. In 2021, demand will outstrip supply, and supply will be insufficient anyway … It is absolutely no risk for anybody who has the capacity to sell,” said a source close to one of the Chinese vaccine makers, who requested anonymity as he was not authorised to speak to the media.
Tao Lina, a Shanghai-based vaccine researcher, said China would still have some advantages when supplying developing countries.
“There was a little bit of pressure for China. If we can be the first to come up with a Covid-19 vaccine, of course it would be great,” he said.
“Usually vaccines are stored at 2-8 degrees Celsius (36-46 Fahrenheit), and that is not possible for [the vaccines developed by] Pfizer and Moderna … there is no problem for ours to be shipped abroad.”
The Pfizer-BioNTech and Moderna products are both made using a revolutionary genetic technique, but need to be stored at very low temperatures – minus 70 and minus 20 respectively – which Tao described as a “big hassle”.Like the Chinese products, the Oxford/AstraZeneca vaccine can also be stored in a regular fridge and Smith agreed that cold chain requirements would be an important consideration when supplying middle-income and developing countries.
“We need multiple vaccines. These may have varying efficacies and, importantly, different cold chain requirements which will affect suitability for use in many situations,” Tao said.
Oxford and AstraZeneca have promised to supply a billion doses of their product to low and middle-income countries, including 400 million before the end of 2021.
Regarding China’s production capacity, Tao said that China was happy to export its vaccines before vaccinating the majority of its population, as speculated by some experts.
“We don’t really need to vaccinate that many people because transmission is low, the production capacity is for the global supply,” said Tao, who is not involved in the vaccines China is developing.
https://www.scmp.com/news/china/diplomacy/article/3112311/covid-19-vaccines-poised-approval-west-what-about-china