Updated 0940 GMT (1740 HKT) July 27, 2022
A version of this story appeared in CNN's Meanwhile in China newsletter, a three-times-a-week update exploring what you need to know about the country's rise and how it impacts the world. Sign up here.
Updated 0940 GMT (1740 HKT) July 27, 2022
A version of this story appeared in CNN's Meanwhile in China newsletter, a three-times-a-week update exploring what you need to know about the country's rise and how it impacts the world. Sign up here.
CNN's Beijing bureau contributed to this report.
Beijing refuses to allow Western shots even though development of homegrown ones lags.
Bloomberg Businessweek
Weeks into a Covid-19 outbreak in Shanghai that brought China’s financial hub to a standstill, the government of President Xi Jinping has demonstrated its willing to go to extremes in its quest to contain the virus. One thing Xi has so far been unwilling to do is deploy a powerful tool against the highly contagious omicron variant: mRNA vaccines. Those shots could reduce the chances of elderly and other vulnerable Chinese getting seriously ill or dying—and possibly help the country transition away from its “Covid Zero” stance.
Lining up the necessary supplies shouldn’t be hard because Shanghai Fosun Pharmaceutical Group Co. in March 2020 agreed to buy a stake of 0.7% in BioNTech SE and to market the mRNA vaccine the German company co-developed with Pfizer Inc. in China. Before the close of that same year, the two companies had arrived at a plan to distribute 100 million doses in China, once they got the green light from the government. Yet the drug regulator has yet to grant approval.
“Worldwide data clearly indicates mRNA is the gold standard,” says Joerg Wuttke, president of the European Union Chamber of Commerce in China, which wrote to the Chinese government in April urging it to allow the shots. “Why waste time and wait, for what?”
As of April 24
Source: Compiled by Bloomberg
The wait, many analysts believe, is for a local company to come up with its own mRNA vaccine. Since the start of the pandemic, Xi’s government has touted self-reliance in fighting Covid, promoting domestic vaccines based on inactivated versions of the virus and barring all foreign ones from the market. Slightly more than 88% of China’s 1.4 billion people have received two doses of those shots.
Opening up to foreign-made mRNA shots risks embarrassing Xi and other officials, says Allison Hills, senior consultant in London with Eradigm Consulting, which advises biotech and pharmaceutical clients. “For them to say now we are accepting BioNTech,” she says, “it’s tantamount to saying ours are not as good.”
Clinical trials have shown the inactivated vaccines from China’s Sinopharm Group Co. and Sinovac Biotech Ltd. to be less effective in stopping infections, though the gap in protecting against severe disease and death is narrower.
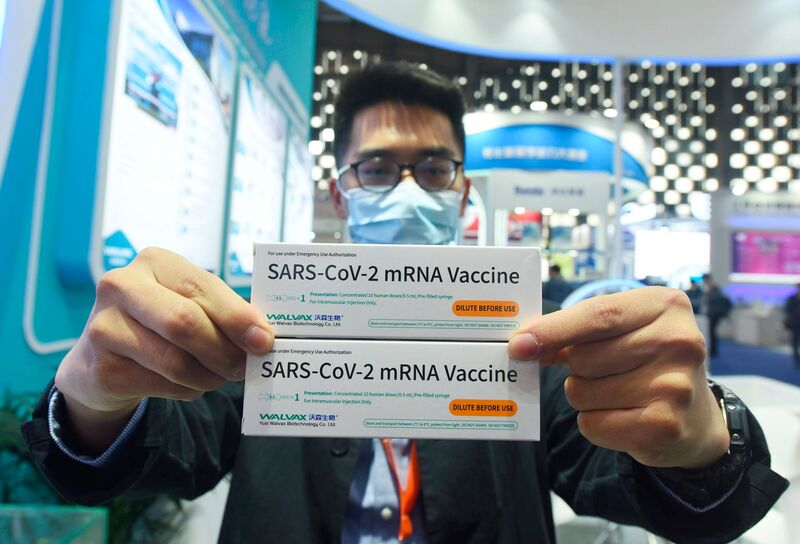
Last year, optimists hoped China’s go-it-alone strategy would lead to the speedy approval of a locally made mRNA vaccine, co-developed by Walvax Biotechnology Co., Suzhou Abogen Bioscience Co., and the Academy of Military Medical Sciences. Upbeat about their chances, the partners invested in a new facility to ramp up production once Beijing gave the green light, with state media reporting production would start by August 2021. However, results of early trials were disappointing and the vaccine is unlikely to reach the market before the end of 2022, according to Bloomberg Intelligence.
One reason for the delay could be the different approach the Walvax group took. Unlike the shots from Pfizer-BioNTech and Moderna Inc., the Chinese vaccine targets a part of the coronavirus spike protein that binds to cells in the body, according to Sam Fazeli, senior pharmaceutical analyst with Bloomberg Intelligence in London. For vaccine developers, focusing on this smaller area, called the receptor binding domain, can reduce costs and may facilitate manufacturing. It can also add uncertainty, given that this particular domain is a focal point for mutations in newer variants.
“Walvax’s problem is the majority of the mutations with omicron are in that receptor binding domain,” says Fazeli. “The risk is that their vaccine’s efficacy is likely to be much more compromised compared to other vaccines.”
Walvax, which didn’t respond to a request for comment regarding the design of the mRNA vaccine it co-developed with Abogen, says it has partnered with a Shanghai-based biotech startup called RNACure Biopharma to develop another mRNA vaccine targeting variants, including omicron. This one encodes a full-length spike protein that covers major mutations from variants. The companies are seeking approvals to begin human testing.
Walvax’s difficulties have raised the stakes for other Chinese companies working on vaccines using the same technology. In early April, China gave permission for CanSino Biologics Inc. and CSPC Pharmaceutical Group Ltd. to each begin first-phase trials for their mRNA shots.
Other drugmakers are further along: Shanghai-based Stemirna Therapeutics Co. has tested an mRNA candidate in Laos and plans further testing in Brazil. It hopes to get emergency-use approval in Southeast Asia and South America. Beijing-based AIM Vaccine Co., which has a Phase 2 trial of its mRNA vaccine under way in China, expects to apply for conditional approval by the end of the year, according to spokesperson Lingna Ding.
Read more: China Crushed Covid. But Covid Zero Could Crush China
Early data from Hong Kong’s winter omicron wave show why mRNA vaccines could be valuable in China. A preprint study by researchers at the University of Hong Kong in late March concluded that two doses of the Sinovac vaccine underperformed BioNTech’s shots, especially among the elderly. For prevention of severe disease, BioNTech’s vaccine effectiveness in people 80 and older was 84.5%, compared with just 60.2% for Sinovac; for protection against death, there was a gap of about 20 percentage points, with BioNTech at 88.2% and Sinovac at 66.8%.
The study, which was funded by the Chinese government, found no significant gap for those who had received three doses. That cohort, however, was small, as only about 10% of seniors had received boosters and government vaccination teams dispatched to nursing homes—sites of the worst outbreaks—only offered Sinovac shots.
Another study by Hong Kong researchers, published in January in the journal Nature, concluded that governments primarily using Sinovac’s vaccines should consider mRNA vaccine boosters in response to the spread of omicron.
Given the strong performance of mRNA vaccines, providing them as boosters should help people who have received two doses of Chinese shots, says Jyoti Somani, senior consultant for the Division of Infectious Diseases at National University Hospital in Singapore, where the government has approved China’s inactivated-virus vaccines as well as mRNA shots developed elsewhere. “It looks like you are getting a much broader immune response when you mix and match,” she says. “What is clear is that we need both.”
That argument is winning support inside China. Zhong Nanshan, a pulmonologist and influential government adviser on Covid, in March co-authored a road map for China’s reopening that identified better booster coverage, with different vaccine types, as essential. Ding Sheng, dean of Tsinghua University’s School of Pharmaceutical Sciences, in March said that existing Chinese vaccines weren’t protective enough against omicron and that the government should encourage companies to introduce more effective shots.
Fosun’s chief executive officer, Wu Yifang, told reporters at a briefing on March 23 that China’s drug regulator is still weighing whether to approve the BioNTech shot. Authorities in February gave the green light to Pfizer’s antiviral treatment, Paxlovid, filling a need because Chinese drugmakers don’t have any antivirals of their own. In the same way, regulators might eventually lose patience with Chinese vaccine makers and open the door to BioNTech’s shots.
With no mRNA vaccines of any kind on the horizon, Chinese health officials may have to focus on better deploying the shots now available, targeting vaccine holdouts, especially among seniors, and improving booster rates. Approximately half the population has received booster shots. That compares with about 30% in the U.S. “Any of the vaccines would be a good thing,” says Colin Pouton, a professor at the Monash Institute of Pharmaceutical Sciences in Melbourne.
The Covid Zero policy is making that more difficult, with millions of residents stuck in their homes in Shanghai and other cities. “We have economic damage, we have social tension, we have basically a whack-a-mole outlook,” says Wuttke. “Two years have passed and China has no mRNA vaccines to offer.” —Bruce Einhorn
https://healthticket.blogspot.com/2022/05/chinas-bet-on-homegrown-mrna-vaccines.html
https://healthticket.blogspot.com/2022/04/chinas-biggest-covid-failure-is-not.html
https://arisechina.blogspot.com/2022/01/china-has-rejected-worlds-top-mrna.html
https://arisechina.blogspot.com/2022/01/china-rushes-to-develop-mrna-covid-19.html
https://healthticket.blogspot.com/2021/05/15-apr-21-how-china-passed-up-vaccine.html
https://healthticket.blogspot.com/2021/05/top-chinese-official-admits-vaccines.html
A new wave of infections is highlighting the growing cost of China’s zero-tolerance virus policy
Sam Fazeli, and
 |
| Workers from a public service organization deliver vegetables to residents of a Shanghai neighborhood in lockdown on March 29. Photographer: Hector Retamal/AFP/Getty Images |
China’s Covid Zero strategy has been drastic and effective, saving lives and keeping the economy on track. But a new wave of virus cases is highlighting the growing costs of that approach – as well as the perils of any attempt to change it.
Authorities are fighting to curb the spread of the omicron variant among a population that lacks natural immunity and only has access to home-grown vaccines that are less effective than some of the global alternatives. Shanghai – the country’s financial center – is locking down just weeks after the technology hub of Shenzhen was forced to do so.
For the rest of the article refer to the link below ...
Source:
Until now, China has rejected the world's most effective and popular COVID-19 vaccine technology ...
Until now, China has rejected the world's most effective and popular COVID-19 vaccine technology—mRNA jabs—and has instead relied on traditional, inactivated vaccines to achieve its 86.6% fully vaccinated rate. But inactivated vaccines are less effective against Omicron and, analysts suspect, China's exclusive use of the old tech is the primary reason why China's borders remain mostly sealed to the outside world.
But this week a Chinese pharmaceutical company announced it is getting closer to developing a homegrown mRNA vaccine, which may prove a critical step in Beijing accepting the technology, providing a much-needed immunity boost to its 1.4 billion population, and finally reopening its borders.
On Monday, Chinese vaccine maker Walvax Biotech published Phase I clinical data on China’s first homegrown mRNA vaccine showing that the vaccine induced an immune response. Walvax is jointly developing the vaccine with private vaccine maker Suzhou Abogen Biosciences and the Chinese military’s Academy of Military Science, and published the peer-reviewed study in The Lancet medical journal.
In the study, Walvax gave six different groups of 20 people two jabs 28 days apart. One group received a placebo, while the other groups received differing doses—5, 10, 15, 20, or 25 micrograms—of the vaccine to gauge the intensity of immune responses.
The results showed the mRNA jab has an efficacy between 80% and 95%, which is on par with the mRNA vaccine developed by Pfizer and BioNTech—but Bloomberg Intelligence analyst Mia He says those early figures don't tell the whole story.
For one, He told Bloomberg, the Walvax vaccine appears to be more effective in generating antibodies than actually catching the virus when administered in certain concentrations. That finding suggests that unless dosing is extremely precise, getting the jab may not provide more protection than getting COVID-19. Other mRNA vaccines, like BioNTech's, don't have that problem.
Plus, the Walvax vaccine appears to cause more side effects, such as fevers, than Pfizer’s or Moderna’s shots do. But the study remains the first clinical proof that China may have a viable homegrown mRNA jab to distribute to its population.
Chinese authorities, meanwhile, appear to have iced out the country's other mRNA option: the BioNTech jab.
Since March 2020, China’s Fosun Pharma has partnered with Germany’s BioNTech to market and distribute the proven and highly effective mRNA jab in the Chinese market. (Pfizer, which struck an agreement with BioNTech after Fosun did, controls distribution rights to BioNTech’s vaccine in most other countries.)
Fosun Pharma has already distributed jabs to places where it controls distribution rights, including Hong Kong, Macau, and Taiwan, and has reportedly built manufacturing facilities to produce the mRNA vaccine in mainland China.
But Beijing has yet to approve the vaccine for distribution in mainland China, despite BioNTech's mRNA shot being more effective than the inactivated vaccines from makers like Sinopharm and Sinovac that China is currently distributing to its citizens.
Last July, when Walvax's drug was still in development, the company's director of research development, Dr. Tong Xin, told Fortune he was optimistic that an mRNA jab would eventually launch in China because "the vaccine technology has been proved effective." But Walvax still has a long way to go before it can bring its drug to market.
Walvax reports it is currently conducting Phase III clinical trial for its vaccine in Mexico and Indonesia, but it is still recruiting the volunteers it needs to carry out the tests. Finding volunteers is increasingly difficult as global vaccination rates have increased since the pandemic began. The trial requires unvaccinated test subjects.
The stakes may be high for Walvax to complete its trials soon and begin rolling out the jabs.
On Wednesday, Bloomberg reported that Hong Kong’s European Chamber of Commerce expects mainland China—and Hong Kong by proxy—to remain closed off from the world until the country starts administering an mRNA vaccine as a booster to its 1.4 billion citizens. The chamber believes that China may only feel confident in opening its borders once citizens can get boosted with more effective mRNA vaccines.
But the chamber said it expects that process of boosting citizens with mRNA shots to take up to two years, with China and Hong Kong potentially remaining closed until the spring of 2024.
Source:
https://fortune.com/2022/01/26/china-covid-vaccine-mrna-homegrown-walvax-reopen-borders/
Other reads:
https://healthticket.blogspot.com/2022/05/chinas-bet-on-homegrown-mrna-vaccines.html
https://healthticket.blogspot.com/2022/04/chinas-biggest-covid-failure-is-not.html
https://arisechina.blogspot.com/2022/01/china-has-rejected-worlds-top-mrna.html
https://arisechina.blogspot.com/2022/01/china-rushes-to-develop-mrna-covid-19.html
https://healthticket.blogspot.com/2021/05/15-apr-21-how-china-passed-up-vaccine.html
https://healthticket.blogspot.com/2021/05/top-chinese-official-admits-vaccines.html