From one tree to 480 million! Just north of Beijing is Saihanba, the world's largest man-made forest. The "Great Green Wall" acts like an environmental sheild and protects north China from sandstorms.
https://www.youtube.com/watch?v=o5xdIVvFEeo
Friday, 29 September 2017
Thursday, 28 September 2017
Early Death Comes From Drinking Distilled Water
During nearly 19 years of clinical practice I have had the opportunity to observe the health effects of drinking different types of water. Most of you would agree that drinking unfiltered tap water could be hazardous to your health because of things like
- parasites
- chlorine
- fluoride
- dioxins
Many health fanatics, however, are often surprised to hear me say that drinking distilled water on a regular, daily basis is potentially dangerous.
Paavo Airola wrote about the dangers of distilled water in the 1970's when it first became a fad with the health food crowd.
Distillation is the process in which water is boiled, evaporated and the vapour condensed. Distilled water is free of dissolved minerals and, because of this, has the special property of being able to actively absorb toxic substances from the body and eliminate them. Studies validate the benefits of drinking distilled water when one is seeking to cleanse or detoxify the system for short periods of time (a few weeks at a time).
Fasting using distilled water can be dangerous because of the rapid loss of electrolytes (sodium, potassium, chloride) and trace minerals like magnesium, deficiencies of which can cause heart beat irregularities and high blood pressure. Cooking foods in distilled water pulls the minerals out of them and lowers their nutrient value.
Distilled water is an active absorber and when it comes into contact with air, it absorbs carbon dioxide, making it acidic. The more distilled water a person drinks, the higher the body acidity becomes.
According to the U.S. Environmental Protection Agency, "Distilled water, being essentially mineral-free, is very aggressive, in that it tends to dissolve substances with which it is in contact. Notably, carbon dioxide from the air is rapidly absorbed, making the water acidic and even more aggressive. Many metals are dissolved by distilled water."
The most toxic commercial beverages that people consume (i.e. cola beverages and other soft drinks) are made from distilled water. Studies have consistently shown that heavy consumers of soft drinks (with or without sugar) spill huge amounts of calcium, magnesium and other trace minerals into the urine.
The more mineral loss, the greater the risk for osteoporosis, osteoarthritis, hypothyroidism, coronary artery disease, high blood pressure and a long list of degenerative diseases generally associated with premature aging.
A growing number of health care practitioners and scientists from around the world have been advocating the theory that aging and disease is the direct result of the accumulation of acid waste products in the body.
There is a great deal of scientific documentation that supports such a theory. A poor diet may be partially to blame for the waste accumulation.
These and other junk foods can cause the body to become more acidic:
- meats
- sugar
- alcohol
- fried foods
- soft drinks
- processed foods
- white flour products
- dairy products
Stress, whether mental or physical can lead to acid deposits in the body.
There is a correlation between the consumption of soft water (distilled water is extremely soft) and the incidence of cardiovascular disease. Cells, tissues and organs do not like to be dipped in acid and will do anything to buffer this acidity including the removal of minerals from the skeleton and the manufacture of bicarbonate in the blood.
The longer one drinks distilled water, the more likely the development of mineral deficiencies and an acid state.
I have done well over 3000 mineral evaluations using a combination of blood, urine and hair tests in my practice. Almost without exception, people who consume distilled water exclusively, eventually develop multiple mineral deficiencies.
Those who supplement their distilled water intake with trace minerals are not as deficient but still not as adequately nourished in minerals as their non-distilled water drinking counterparts even after several years of mineral supplementation.
The ideal water for the human body should be slightly alkaline and this requires the presence of minerals like
- calcium
- magnesium
Distilled water tends to be acidic and can only be recommended as a way of drawing poisons out of the body. Once this is accomplished, the continued drinking of distilled water is a bad idea.
Water filtered through reverse osmosis tends to be neutral and is acceptable for regular use provided minerals are supplemented.
Water filtered through a solid charcoal filter is slightly alkaline. Ozonation of this charcoal filtered water is ideal for daily drinking. Longevity is associated with the regular consumption of hard water (high in minerals). Disease and early death is more likely to be seen with the long term drinking of distilled water.
Avoid it except in special circumstances.
Zoltan P. Rona MD MSc
http://www.mercola.com/article/water/distilled_water.htm
Labels:
Ageing,
Calcium,
Cardiovascular Health,
Distilled Water,
Dr Mercola,
High Blood Pressure,
Hypothyroidism,
Magnesium,
Minerals,
Osteoarthritis,
Osteoporosis,
Processed Food/Meat,
Water
WHO report: Health Risks from Drinking Demineralised Water - MUST READ
HEALTH RISKS FROM DRINKING
DEMINERALISED WATER
Frantisek Kozisek National Institute of Public Health Czech Republic _______________________________________________________

I. INTRODUCTION
The composition of water varies widely with local geological conditions. Neither groundwater nor surface water has ever been chemically pure H2O, since water contains small amounts of gases, minerals and organic matter of natural origin. The total concentrations of substances dissolved in fresh water considered to be of good quality can be hundreds of mg/L. Thanks to epidemiology and advances in microbiology and chemistry since the 19th century, numerous waterborne disease causative agents have been identified. The knowledge that water may contain some constituents that are undesirable is the point of departure for establishing guidelines and regulations for drinking water quality. Maximum acceptable concentrations of inorganic and organic substances and microorganisms have been established internationally and in many countries to assure the safety of drinking water. The potential effects of totally unmineralised water had not generally been considered, since this water is not found in nature except possibly for rainwater and naturally formed ice. Although rainwater and ice are not used as community drinking water sources in industrialized countries where drinking water regulations were developed, they are used by individuals in some locations. In addition, many natural waters are low in many minerals or soft (low in divalent ions), and hard waters are often artificially softened.
Awareness of the importance of minerals and other beneficial constituents in drinking water has existed for thousands years, being mentioned in the Vedas of ancient India. In the book Rig Veda, the properties of good drinking water were described as follows: “Sheetham (cold to touch), Sushihi (clean), Sivam (should have nutritive value, requisite minerals and trace elements), Istham (transparent), Vimalam lahu Shadgunam (its acid base balance should be within normal limits)” (1). That water may contain desirable substances has received less attention in guidelines and regulations, but an increased awareness of the biological value of water has occurred in the past several decades.
Artificially-produced demineralised waters, first distilled water and later also deionized or reverse osmosis-treated water, had been used mainly for industrial, technical and laboratory purposes. These technologies became more extensively applied in drinking water treatment in the 1960’s as limited drinking water sources in some coastal and inland arid areas could not meet the increasing water demands resulting from increasing populations, higher living standards, development of industry, and mass tourism. Demineralisation of water was needed where the primary or the only abundant water source available was highly mineralized brackish water or sea water. Drinking water supply was also of concern to ocean-going ships, and spaceships as well. Initially, these water treatment methods were not used elsewhere since they were technically exacting and costly.
p 149
In this chapter, demineralised water is defined as water almost or completely free of dissolved minerals as a result of distillation, deionization, membrane filtration (reverse osmosis or nanofiltration), electrodialysis or other technology. The total dissolved solids (TDS) in such water can vary but TDS could be as low as 1 mg/L. The electrical conductivity is generally less than 2 mS/m and may even be lower (<0.1 mS/m). Although the technology had its beginnings in the 1960’s, demineralization was not widely used at that time.
However, some countries focused on public health research in this field, mainly the former USSR where desalination was introduced to produce drinking water in some Central Asian cities. It was clear from the very beginning that desalinated or demineralised water without further enrichment with some minerals might not be fully appropriate for consumption.
There were three reasons for this:
• Demineralised water is highly aggressive and if untreated, its distribution through pipes and storage tanks would not be possible. The aggressive water attacks the water distribution piping and leaches metals and other materials from the pipes and associated plumbing materials.
• Distilled water has poor taste characteristics.
• Preliminary evidence was available that some substances present in water could have beneficial effects on human health as well as adverse effects. For example, experience with artificially fluoridated water showed a decrease in the incidence of tooth caries, and some epidemiological studies in the 1960’s reported lower morbidity and mortality from some cardiovascular diseases in areas with hard water.
Therefore, researchers focused on two issues:
1.) what are the possible adverse health effects of demineralised water, and
2.) what are the minimum and the desirable or optimum contents of the relevant substances (e.g., minerals) in drinking water needed to meet both technical and health considerations.
The traditional regulatory approach, which was previously based on limiting the health risks from excessive concentrations of toxic substances in water, now took into account possible adverse effects due to the deficiency of certain constituents.
At one of the working meetings for preparation of guidelines for drinking water quality, the World Health Organization (WHO) considered the issue of the desired or optimum mineral composition of desalinated drinking water by focusing on the possible adverse health effects of removing some substances that are naturally present in drinking water (2). In the late 1970’s, the WHO also commissioned a study to provide background information for issuing guidelines for desalinated water. That study was conducted by a team of researchers of the A.N. Sysin Institute of General and Public Hygiene and USSR Academy of Medical Sciences under the direction of Professor Sidorenko and Dr. Rakhmanin.
The final report, published in 1980 as an internal working document (3), concluded that “not only does completely demineralised water (distillate) have unsatisfactory organoleptic properities, but it also has a definite adverse influence on the animal and human organism”.
After evaluating the available health, organoleptic, and other information, the team recommended that demineralised water contain 1.) a minimum level for dissolved salts (100 mg/L), bicarbonate ion (30 mg/L), and calcium (30 mg/L); 2.) an optimum level for total dissolved salts (250-500 mg/L for chloride-sulfate water and 250-500 mg/L for bicarbonate water); 3.) a maximum level for alkalinity (6.5 meq/l), sodium (200 mg/L), boron (0.5 mg/L), and bromine (0.01 mg/L). Some of these recommendations are discussed in greater detail in this chapter.
During the last three decades, desalination has become a widely practiced technique in providing new fresh water supplies. There are more than 11 thousand desalination plants all over the world with an overall production of more than 6 billion gallons of desalinated water per day (Cotruvo, in this book). In some regions such as the Middle East and Western Asia more than half
p 150
of the drinking water is produced in this way. Desalinated waters are commonly further treated by adding chemical constituents such as calcium carbonate or limestone, or blended with small volumes of more mineral-rich waters to improve their taste and reduce their aggressiveness to the distribution network as well as plumbing materials. However, desalinated waters may vary widely in composition, especially in terms of the minimum TDS content. Numerous facilities were developed without compliance with any uniform guidelines regarding minimum mineral content for final product quality.
The potential for adverse health effects from long term consumption of demineralised water is of interest not only in countries lacking adequate fresh water, but also in countries where some types of home water treatment systems are widely used or where some types of bottled water are consumed. Some natural mineral waters, in particular glacial mineral waters, are low in TDS (less than 50 mg/l) and in some countries, even distilled bottled water has been supplied for drinking purposes. Otherbrands of bottled water are produced by demineralising fresh water and then adding minerals for desirable taste. Persons consuming certain types of water may not be receiving the additional minerals that would be present in more highly mineralized waters. Consequently, the exposures and risks should be considered not only at the community level, but also at the individual or family level.
II. HEALTH RISKS FROM CONSUMPTION OF DEMINERALISED OR LOW-MINERAL WATER
Knowledge of some effects of consumption of demineralised water is based on experimental and observational data. Experiments have been conducted in laboratory animals and human volunteers, and observational data have been obtained from populations supplied with desalinated water, individuals drinking reverse osmosis-treated demineralised water, and infants given beverages prepared with distilled water. Because limited information is available from these studies, we should also consider the results of epidemiological studies where health effects were compared for populations using low-mineral (soft) water and more mineral-rich waters. Demineralised water that has not been remineralised is considered an extreme case of low-mineral or soft water because it contains only small amounts of dissolved minerals such as calcium and magnesium that are the major contributors to hardness.
The possible adverse consequences of low mineral content water consumption are discussed in the following categories:
• Direct effects on the intestinal mucous membrane, metabolism and mineral homeostasis or other body functions.
• Little or no intake of calcium and magnesium from low-mineral water. • Low intake of other essential elements and microelements.
• Loss of calcium, magnesium and other essential elements in prepared food.
• Possible increased dietary intake of toxic metals.
1. Direct effects of low mineral content water on the intestinal mucous membrane, metabolism and mineral homeostasis or other body functions
Distilled and low mineral content water (TDS < 50 mg/L) can have negative taste characteristics to which the consumer may adapt with time. This water is also reported to be less thirst quenching (3). Although these are not considered to be health effects, they should be taken into account when considering the suitability of low mineral content water for human consumption.
p 151
Poor organoleptic and thirst-quenching characteristics may affect the amount of water consumed or cause persons to seek other, possibly less satisfactory water sources.
Williams (4) reported that distilled water introduced into the intestine caused abnormal changes in epithelial cells of rats, possibly due to osmotic shock. However, the same conclusions were not reached by Schumann et al. (5) in a more recent study based on 14-day experiments in rats. Histology did not reveal any signs of erosion, ulceration or inflammation in the oesophagus, stomach and jejunum. Altered secretory function in animals (i.e., increased secretion and acidity of gastric juice) and altered stomach muscle tone were reported in studies for WHO (3), but currently available data have not unambiguously demonstrated a direct negative effect of low mineral content water on the gastrointestinal mucous membrane.
It has been adequately demonstrated that consuming water of low mineral content has a negative effect on homeostasis mechanisms, compromising the mineral and water metabolism in the body. An increase in urine output (i.e., increased diuresis) is associated with an increase in excretion of major intra- and extracellular ions from the body fluids, their negative balance, and changes in body water levels and functional activity of some body water management-dependent hormones.
Experiments in animals, primarily rats, for up to one-year periods have repeatedly shown that the intake of distilled water or water with TDS ≤ 75 mg/L leads to:
1.) increased water intake, diuresis, extracellular fluid volume, and serum concentrations of sodium (Na) and chloride (Cl) ions and their increased elimination from the body, resulting in an overall negative balance.., and
2.) lower volumes of red cells and some other hematocrit changes (3).
Although Rakhmanin et al. (6) did not find mutagenic or gonadotoxic effects of distilled water, they did report decreased secretion of tri-iodothyronine and aldosterone, increased secretion of cortisol, morphological changes in the kidneys including a more pronounced atrophy of glomeruli, and swollen vascular endothelium limiting the blood flow.
Reduced skeletal ossification was also found in rat foetuses whose dams were given distilled water in a one-year study. Apparently the reduced mineral intake from water was not compensated by their diets, even if the animals were kept on standardized diet that was physiologically adequate in caloric value, nutrients and salt composition.
Results of experiments in human volunteers evaluated by researchers for the WHO report (3) are in agreement with those in animal experiments and suggest the basic mechanism of the effects of water low in TDS (e.g. < 100 mg/L) on water and mineral homeostasis.
Low-mineral water markedly:
1.) increased diuresis (almost by 20%, on average), body water volume, and serum sodium concentrations,
2.) decreased serum potassium concentration, and
3.) increased the elimination of sodium, potassium, chloride, calcium and magnesium ions from the body.
It was thought that low-mineral water acts on osmoreceptors of the gastrointestinal tract, causing an increased flow of sodium ions into the intestinal lumen and slight reduction in osmotic pressure in the portal venous system with subsequent enhanced release of sodium into the blood as an adaptation response.
This osmotic change in the blood plasma results in the redistribution of body water; that is, there is an increase in the total extracellular fluid volume and the transfer of water from erythrocytes and interstitial fluid into the plasma and between intracellular and interstitial fluids.
In response to the changed plasma volume, baroreceptors and volume receptors in the bloodstream are activated, inducing a decrease in aldosterone release and thus an increase in sodium elimination. Reactivity of the volume receptors in the vessels may result in a decrease in ADH release and an enhanced diuresis.
The German Society for Nutrition reached similar conclusions about the effects of distilled water and warned the public against drinking it (7). The warning was published in response to the German edition of The Shocking Truth About Water (8), whose authors recommended drinking distilled water instead of "ordinary" drinking water. The Society in its position paper (7) explains that water in the human body always contains
p 152
Illustrative of such short-term exposures are cases in the Czech and Slovak populations who began using reverse osmosis-based systems for final treatment of drinking water at their home taps in 2000-2002. Within several weeks or months various complaints suggestive of acute magnesium (and possibly calcium) deficiency were reported (34). The complaints included cardiovascular disorders, tiredness, weakness or muscular cramps and were essentially the same symptoms listed in the warning of the German Society for Nutrition (7).
3. Low intake of some essential elements and microelements from low-mineral water
Although drinking water, with some rare exceptions, is not the major source of essential elements for humans, its contribution may be important for several reasons. The modern diet of many people may not be an adequate source of minerals and microelements. In the case of borderline deficiency of a given element, even the relatively low intake of the element with drinking water may play a relevant protective role. This is because the elements are usually present in water as free ions and therefore, are more readily absorbed from water compared to food where they are mostly bound to other substances.
These results were found in a 6-month experiment in which rats were randomized into 4 groups and given: a.) tap water, b.) low-mineral water, c.) low-mineral water supplemented with iodide, cobalt, copper, manganese, molybdenum, zinc and fluoride in tap water, d.) low-mineral water supplemented with the same elements but at ten times higher concentrations. Furthermore, a negative effect on the blood formation process was found to be associated with non-supplemented demineralised water. The mean hemoglobin content of red blood cells was as much as 19% lower in the animals that received non-supplemented demineralised water compared to that in animals
Recent epidemiological studies of an ecologic design among Russian populations supplied with water varying in TDS suggest that low-mineral drinking water may be a risk factor for hypertension and coronary heart disease, gastric and duodenal ulcers, chronic gastritis, goitre, pregnancy complications and several complications in newborns and infants, including jaundice, anemia, fractures and growth disorders (36). However, it is not clear whether the effects observed in these studies are due to the low content of calcium and magnesium or other essential elements, or due to other factors.
4. High loss of calcium, magnesium and other essential elements in food prepared in low-mineral water
When used for cooking, soft water was found to cause substantial losses of all essential elements from food (vegetables, meat, cereals). Such losses may reach up to 60 % for magnesium and calcium or even more for some other microelements (e.g., copper 66 %, manganese 70 %, cobalt 86 %). In contrast, when hard water is used for cooking, the loss of these elements is much lower, and in some cases, an even higher calcium content was reported in food as a result of cooking (38-41).
Since most nutrients are ingested with food, the use of low-mineral water for cooking and processing food may cause a marked deficiency in total intake of some essential elements that was much higher than expected with the use of such water for drinking only. The current diet of many persons usually does not provide all necessary elements in sufficient quantities, and therefore, any factor that results in the loss of essential elements and nutrients during the processing and preparation of food could be detrimental for them.
5. Possible increased dietary intake of toxic metals
Increased risk from toxic metals may be posed by low-mineral water in two ways:
Low-mineralized water is unstable and therefore, highly aggressive to materials with which it comes into contact. Such water more readily dissolves metals and some organic substances from pipes, coatings, storage tanks and containers, hose lines and fittings, being incapable of forming low-absorbable complexes with some toxic substances and thus reducing their negative effects.
Among eight outbreaks of chemical poisoning from drinking water reported in the USA in 1993-1994, there were three cases of lead poisoning in infants who had blood-lead levels of 15 µg/dL, 37 µg/dL, and 42 µg/dL. The level of concern is 10 µg/dL. For all three cases, lead had leached from brass fittings and lead-soldered seams in drinking water storage tanks.
Calcium and, to a lesser extent, magnesium in water and food are known to have antitoxic activity. They can help prevent the absorption of some toxic elements such as lead and cadmium from the intestine into the blood, either via direct reaction leading to formation of an unabsorbable compound or via competition for binding sites (44-50). Although this protective effect is limited, it should not be dismissed. Populations supplied with low-mineral water may be at a higher risk in terms of adverse effects from exposure to toxic substances compared to populations supplied with water of average mineralization and hardness.
6. Possible bacterial contamination of low-mineral water
All water is prone to bacterial contamination in the absence of a disinfectant residual either at source or as a result of microbial re-growth in the pipe system after treatment. Re-growth may also occur in desalinated water. Bacterial re-growth within the pipe system is encouraged by higher initial temperatures, higher temperatures of water in the distribution system due to hot climates, lack of a residual disinfectant, and possibly greater availability of some nutrients due to the aggressive nature of the water to materials in contact with it.
III. DESIRABLE MINERAL CONTENT OF DEMINERALISED DRINKING WATER
The corrosive nature of demineralised water and potential health risks related to the distribution and consumption of low TDS water has led to recommendations of the minimum and optimum mineral content in drinking water and then, in some countries, to the establishment of obligatory values in the respective legislative or technical regulations for drinking water quality. Organoleptic characteristics and thirst-quenching capacity were also considered in the recommendations. For example, human volunteer studies (3) showed that the water temperatures of 15-350 C best satisfied physiological needs. Water temperatures above 350 or below 150 C
p 156
resulted in a reduction in water consumption. Water with a TDS of 25-50 mg/L was described tasteless (3).
1. The 1980 WHO report
Salts are leached from the body under the influence of drinking water with a low TDS. Because adverse effects such as altered water-salt balance were observed not only in completely desalinated water but also in water with TDS between 50 and 75 mg/L, the team that prepared the 1980 WHO report (3) recommended that the minimum TDS in drinking water should be 100 mg/L. The team also recommended that the optimum TDS should be about 200-400 mg/L for chloride-sulphate waters and 250-500 mg/L for bicarbonate waters (WHO 1980).
More recent studies have provided additional information about minimum and optimum levels of minerals that should be in demineralised water. For example, the effect of drinking water of different hardness on the health status of women aged from 20 to 49 years was the subject of two cohort epidemiological studies (460 and 511 women) in four South Siberian cities (55, 56).
• For magnesium, a minimum of 10 mg/L (33, 56) and an optimum of about 20-30 mg/L (49, 57);
p 157
• For calcium, a minimum of 20 mg/L (56) and an optimum of about 50 (40-80) mg/L (57, 58);
• For total water hardness, the sum of calcium and magnesium should be 2 to 4 mmol/L (37, 50, 59, 60).
At these concentrations, minimum or no adverse health effects were observed. The maximum protective or beneficial health effects of drinking water appeared to occur at the
estimated desirable or optimum concentrations. The recommended magnesium levels were based on cardiovascular system effects, while changes in calcium metabolism and ossification were used as a basis for the recommended calcium levels. The upper limit of the hardness optimal range was derived from data that showed a higher risk of gall stones, kidney stones, urinary stones, arthrosis and arthropathies in populations supplied with water of hardness higher than 5 mmol/L. Long-term intake of drinking water was taken into account in estimating these concentrations. For short-term therapeutic indications of some waters, higher concentrations of these elements may be considered.
The WHO in the 2nd edition of Guidelines for Drinking-water Quality (61) evaluated calcium and magnesium in terms of water hardness but did not recommend either minimum levels or maximum limits for calcium, magnesium, or hardness.The first European Directive (62) established a requirement for minimum hardness for softened or desalinated water (≥ 60 mg/L as calcium or equivalent cations). This requirement appeared obligatorily in the national legislations of all EEC members, but this Directive expired in December 2003 when a new Directive (63) became effective. The new Directive does not contain a requirement for calcium, magnesium, or water hardness levels. On the other hand, it does not prevent member states from implementing such a requirement into their national legislation.
V. CONCLUSIONS
Drinking water should contain minimum levels of certain essential minerals (and other components such as carbonates). Unfortunately, over the two past decades, little research attention has been given to the beneficial or protective effects of drinking water substances. The main focus has been on the toxicological properties of contaminants. Nevertheless, some studies have attempted to define the minimum content of essential elements or TDS in drinking water, and some countries have included requirements or guidelines for selected substances in their drinking water regulations. The issue is relevant not only where drinking water is obtained by desalination (if not adequately re-mineralised) but also where home treatment or central water treatment reduces the content of important minerals and low-mineral bottled water is consumed.
Drinking water manufactured by desalination is stabilized with some minerals, but this is usually not the case for water demineralised as a result of household treatment. Even when
stabilized, the final composition of some waters may not be adequate in terms of providing health benefits. Although desalinated waters are supplemented mainly with calcium (lime) or other carbonates, they may be deficient in magnesium and other microelements such as fluorides and potassium. Furthermore, the quantity of calcium that is supplemented is based on technical considerations (i.e., reducing the aggressiveness) rather than on health concerns. Possibly none of the commonly used ways of re-mineralization could be considered optimum, since the water does not contain all of its beneficial components. Current methods of stabilization are primarily intended to decrease the corrosive effects of demineralised water.
Sufficient evidence is now available to confirm the health consequences from drinking water deficient in calcium or magnesium. Many studies show that higher water magnesium is related to decreased risks for CVD and especially for sudden death from CVD. This relationship has been independently described in epidemiological studies with different study designs, performed in different areas, different populations, and at different times. The consistent epidemiological observations are supported by the data from autopsy, clinical, and animal studies. Biological plausibility for a protective effect of magnesium is substantial, but the specificity is less evident due to the multifactorial aetiology of CVD. In addition to an increased risk of sudden death, it has been suggested that intake of water low in magnesium may be associated with a higher risk of motor neuronal disease, pregnancy disorders (so-called preeclampsia), sudden death in infants, and some types of cancer. Recent studies suggest that the intake of soft water, i.e. water low in calcium, is associated with a higher risk of fracture in children, certain neurodegenerative
p 159
diseases, pre-term birth and low weight at birth and some types of cancer. Furthermore, the possible role of water calcium in the development of CVD cannot be excluded.
International and national authorities responsible for drinking water quality should consider guidelines for desalination water treatment, specifying the minimum content of the relevant elements such as calcium and magnesium and TDS. If additional research is required to establish guidelines, authorities should promote targeted research in this field to elaborate the health benefits. If guidelines are established for substances that should be in deminerialised water, authorities should ensure that the guidelines also apply to uses of certain home treatment devices and bottled waters.
Health Risk from Drinking Demineralized Water (PDF Download Available). Available from: https://www.researchgate.net/publication/252043662_Health_Risk_from_Drinking_Demineralized_Water [accessed Sep 28, 2017].
http://www.who.int/water_sanitation_health/dwq/nutrientschap12.pdf
References:
For references referred to in the above article, please go to the above external links.
Frantisek Kozisek National Institute of Public Health Czech Republic _______________________________________________________

I. INTRODUCTION
The composition of water varies widely with local geological conditions. Neither groundwater nor surface water has ever been chemically pure H2O, since water contains small amounts of gases, minerals and organic matter of natural origin. The total concentrations of substances dissolved in fresh water considered to be of good quality can be hundreds of mg/L. Thanks to epidemiology and advances in microbiology and chemistry since the 19th century, numerous waterborne disease causative agents have been identified. The knowledge that water may contain some constituents that are undesirable is the point of departure for establishing guidelines and regulations for drinking water quality. Maximum acceptable concentrations of inorganic and organic substances and microorganisms have been established internationally and in many countries to assure the safety of drinking water. The potential effects of totally unmineralised water had not generally been considered, since this water is not found in nature except possibly for rainwater and naturally formed ice. Although rainwater and ice are not used as community drinking water sources in industrialized countries where drinking water regulations were developed, they are used by individuals in some locations. In addition, many natural waters are low in many minerals or soft (low in divalent ions), and hard waters are often artificially softened.
Awareness of the importance of minerals and other beneficial constituents in drinking water has existed for thousands years, being mentioned in the Vedas of ancient India. In the book Rig Veda, the properties of good drinking water were described as follows: “Sheetham (cold to touch), Sushihi (clean), Sivam (should have nutritive value, requisite minerals and trace elements), Istham (transparent), Vimalam lahu Shadgunam (its acid base balance should be within normal limits)” (1). That water may contain desirable substances has received less attention in guidelines and regulations, but an increased awareness of the biological value of water has occurred in the past several decades.
Artificially-produced demineralised waters, first distilled water and later also deionized or reverse osmosis-treated water, had been used mainly for industrial, technical and laboratory purposes. These technologies became more extensively applied in drinking water treatment in the 1960’s as limited drinking water sources in some coastal and inland arid areas could not meet the increasing water demands resulting from increasing populations, higher living standards, development of industry, and mass tourism. Demineralisation of water was needed where the primary or the only abundant water source available was highly mineralized brackish water or sea water. Drinking water supply was also of concern to ocean-going ships, and spaceships as well. Initially, these water treatment methods were not used elsewhere since they were technically exacting and costly.
p 149
In this chapter, demineralised water is defined as water almost or completely free of dissolved minerals as a result of distillation, deionization, membrane filtration (reverse osmosis or nanofiltration), electrodialysis or other technology. The total dissolved solids (TDS) in such water can vary but TDS could be as low as 1 mg/L. The electrical conductivity is generally less than 2 mS/m and may even be lower (<0.1 mS/m). Although the technology had its beginnings in the 1960’s, demineralization was not widely used at that time.
However, some countries focused on public health research in this field, mainly the former USSR where desalination was introduced to produce drinking water in some Central Asian cities. It was clear from the very beginning that desalinated or demineralised water without further enrichment with some minerals might not be fully appropriate for consumption.
There were three reasons for this:
• Demineralised water is highly aggressive and if untreated, its distribution through pipes and storage tanks would not be possible. The aggressive water attacks the water distribution piping and leaches metals and other materials from the pipes and associated plumbing materials.
• Distilled water has poor taste characteristics.
• Preliminary evidence was available that some substances present in water could have beneficial effects on human health as well as adverse effects. For example, experience with artificially fluoridated water showed a decrease in the incidence of tooth caries, and some epidemiological studies in the 1960’s reported lower morbidity and mortality from some cardiovascular diseases in areas with hard water.
Therefore, researchers focused on two issues:
1.) what are the possible adverse health effects of demineralised water, and
2.) what are the minimum and the desirable or optimum contents of the relevant substances (e.g., minerals) in drinking water needed to meet both technical and health considerations.
The traditional regulatory approach, which was previously based on limiting the health risks from excessive concentrations of toxic substances in water, now took into account possible adverse effects due to the deficiency of certain constituents.
At one of the working meetings for preparation of guidelines for drinking water quality, the World Health Organization (WHO) considered the issue of the desired or optimum mineral composition of desalinated drinking water by focusing on the possible adverse health effects of removing some substances that are naturally present in drinking water (2). In the late 1970’s, the WHO also commissioned a study to provide background information for issuing guidelines for desalinated water. That study was conducted by a team of researchers of the A.N. Sysin Institute of General and Public Hygiene and USSR Academy of Medical Sciences under the direction of Professor Sidorenko and Dr. Rakhmanin.
The final report, published in 1980 as an internal working document (3), concluded that “not only does completely demineralised water (distillate) have unsatisfactory organoleptic properities, but it also has a definite adverse influence on the animal and human organism”.
After evaluating the available health, organoleptic, and other information, the team recommended that demineralised water contain 1.) a minimum level for dissolved salts (100 mg/L), bicarbonate ion (30 mg/L), and calcium (30 mg/L); 2.) an optimum level for total dissolved salts (250-500 mg/L for chloride-sulfate water and 250-500 mg/L for bicarbonate water); 3.) a maximum level for alkalinity (6.5 meq/l), sodium (200 mg/L), boron (0.5 mg/L), and bromine (0.01 mg/L). Some of these recommendations are discussed in greater detail in this chapter.
During the last three decades, desalination has become a widely practiced technique in providing new fresh water supplies. There are more than 11 thousand desalination plants all over the world with an overall production of more than 6 billion gallons of desalinated water per day (Cotruvo, in this book). In some regions such as the Middle East and Western Asia more than half
p 150
of the drinking water is produced in this way. Desalinated waters are commonly further treated by adding chemical constituents such as calcium carbonate or limestone, or blended with small volumes of more mineral-rich waters to improve their taste and reduce their aggressiveness to the distribution network as well as plumbing materials. However, desalinated waters may vary widely in composition, especially in terms of the minimum TDS content. Numerous facilities were developed without compliance with any uniform guidelines regarding minimum mineral content for final product quality.
The potential for adverse health effects from long term consumption of demineralised water is of interest not only in countries lacking adequate fresh water, but also in countries where some types of home water treatment systems are widely used or where some types of bottled water are consumed. Some natural mineral waters, in particular glacial mineral waters, are low in TDS (less than 50 mg/l) and in some countries, even distilled bottled water has been supplied for drinking purposes. Otherbrands of bottled water are produced by demineralising fresh water and then adding minerals for desirable taste. Persons consuming certain types of water may not be receiving the additional minerals that would be present in more highly mineralized waters. Consequently, the exposures and risks should be considered not only at the community level, but also at the individual or family level.
II. HEALTH RISKS FROM CONSUMPTION OF DEMINERALISED OR LOW-MINERAL WATER
Knowledge of some effects of consumption of demineralised water is based on experimental and observational data. Experiments have been conducted in laboratory animals and human volunteers, and observational data have been obtained from populations supplied with desalinated water, individuals drinking reverse osmosis-treated demineralised water, and infants given beverages prepared with distilled water. Because limited information is available from these studies, we should also consider the results of epidemiological studies where health effects were compared for populations using low-mineral (soft) water and more mineral-rich waters. Demineralised water that has not been remineralised is considered an extreme case of low-mineral or soft water because it contains only small amounts of dissolved minerals such as calcium and magnesium that are the major contributors to hardness.
The possible adverse consequences of low mineral content water consumption are discussed in the following categories:
• Direct effects on the intestinal mucous membrane, metabolism and mineral homeostasis or other body functions.
• Little or no intake of calcium and magnesium from low-mineral water. • Low intake of other essential elements and microelements.
• Loss of calcium, magnesium and other essential elements in prepared food.
• Possible increased dietary intake of toxic metals.
1. Direct effects of low mineral content water on the intestinal mucous membrane, metabolism and mineral homeostasis or other body functions
Distilled and low mineral content water (TDS < 50 mg/L) can have negative taste characteristics to which the consumer may adapt with time. This water is also reported to be less thirst quenching (3). Although these are not considered to be health effects, they should be taken into account when considering the suitability of low mineral content water for human consumption.
p 151
Poor organoleptic and thirst-quenching characteristics may affect the amount of water consumed or cause persons to seek other, possibly less satisfactory water sources.
Williams (4) reported that distilled water introduced into the intestine caused abnormal changes in epithelial cells of rats, possibly due to osmotic shock. However, the same conclusions were not reached by Schumann et al. (5) in a more recent study based on 14-day experiments in rats. Histology did not reveal any signs of erosion, ulceration or inflammation in the oesophagus, stomach and jejunum. Altered secretory function in animals (i.e., increased secretion and acidity of gastric juice) and altered stomach muscle tone were reported in studies for WHO (3), but currently available data have not unambiguously demonstrated a direct negative effect of low mineral content water on the gastrointestinal mucous membrane.
It has been adequately demonstrated that consuming water of low mineral content has a negative effect on homeostasis mechanisms, compromising the mineral and water metabolism in the body. An increase in urine output (i.e., increased diuresis) is associated with an increase in excretion of major intra- and extracellular ions from the body fluids, their negative balance, and changes in body water levels and functional activity of some body water management-dependent hormones.
Experiments in animals, primarily rats, for up to one-year periods have repeatedly shown that the intake of distilled water or water with TDS ≤ 75 mg/L leads to:
1.) increased water intake, diuresis, extracellular fluid volume, and serum concentrations of sodium (Na) and chloride (Cl) ions and their increased elimination from the body, resulting in an overall negative balance.., and
2.) lower volumes of red cells and some other hematocrit changes (3).
Although Rakhmanin et al. (6) did not find mutagenic or gonadotoxic effects of distilled water, they did report decreased secretion of tri-iodothyronine and aldosterone, increased secretion of cortisol, morphological changes in the kidneys including a more pronounced atrophy of glomeruli, and swollen vascular endothelium limiting the blood flow.
Reduced skeletal ossification was also found in rat foetuses whose dams were given distilled water in a one-year study. Apparently the reduced mineral intake from water was not compensated by their diets, even if the animals were kept on standardized diet that was physiologically adequate in caloric value, nutrients and salt composition.
Results of experiments in human volunteers evaluated by researchers for the WHO report (3) are in agreement with those in animal experiments and suggest the basic mechanism of the effects of water low in TDS (e.g. < 100 mg/L) on water and mineral homeostasis.
Low-mineral water markedly:
1.) increased diuresis (almost by 20%, on average), body water volume, and serum sodium concentrations,
2.) decreased serum potassium concentration, and
3.) increased the elimination of sodium, potassium, chloride, calcium and magnesium ions from the body.
It was thought that low-mineral water acts on osmoreceptors of the gastrointestinal tract, causing an increased flow of sodium ions into the intestinal lumen and slight reduction in osmotic pressure in the portal venous system with subsequent enhanced release of sodium into the blood as an adaptation response.
This osmotic change in the blood plasma results in the redistribution of body water; that is, there is an increase in the total extracellular fluid volume and the transfer of water from erythrocytes and interstitial fluid into the plasma and between intracellular and interstitial fluids.
In response to the changed plasma volume, baroreceptors and volume receptors in the bloodstream are activated, inducing a decrease in aldosterone release and thus an increase in sodium elimination. Reactivity of the volume receptors in the vessels may result in a decrease in ADH release and an enhanced diuresis.
The German Society for Nutrition reached similar conclusions about the effects of distilled water and warned the public against drinking it (7). The warning was published in response to the German edition of The Shocking Truth About Water (8), whose authors recommended drinking distilled water instead of "ordinary" drinking water. The Society in its position paper (7) explains that water in the human body always contains
p 152
electrolytes (e.g. potassium and sodium) at certain concentrations controlled by the body. Water resorption by the intestinal epithelium is also enabled by sodium transport. If distilled water is ingested, the intestine has to add electrolytes to this water first, taking them from the body reserves. Since the body never eliminates fluid in form of "pure" water but always together with salts, adequate intake of electrolytes must be ensured.
Ingestion of distilled water leads to the dilution of the electrolytes dissolved in the body water. Inadequate body water redistribution between compartments may compromise the function of vital organs. Symptoms at the very beginning of this condition include tiredness, weakness and headache; more severe symptoms are muscular cramps and impaired heart rate.
2. Little or no intake of calcium and magnesium from low-mineral water
Calcium and magnesium are both essential elements. Calcium is a substantial component of bones and teeth. In addition, it plays a role in neuromuscular excitability (i.e., decreases it), the proper function of the conducting myocardial system, heart and muscle contractility, intracellular information transmission and the coagulability of blood. Magnesium plays an important role as a cofactor and activator of more than 300 enzymatic reactions including glycolysis, ATP metabolism, transport of elements such as sodium, potassium, and calcium through membranes, synthesis of proteins and nucleic acids, neuromuscular excitability and muscle contraction.
Additional evidence comes from animal experiments and clinical observations in several countries. Animals given zinc or magnesium dosed in their drinking water had a significantly higher concentration of these elements in the serum than animals given the same elements in much higher amounts with food and provided with low-mineral water to drink. Based on the results of experiments and clinical observations of mineral deficiency in patients whose intestinal absorption did not need to be taken into account and who received balanced intravenous nutrition diluted with distilled water, Robbins and Sly (9) presumed that intake of low-mineral water was responsible for an increased elimination of minerals from the body.
Regular intake of low-mineral content water could be associated with the progressive evolution of the changes discussed above, possibly without manifestation of symptoms or causal symptoms over the years. Nevertheless, severe acute damage, such as hyponatremic shock or delirium, may occur following intense physical efforts and ingestion of several litres of low-mineral water (10). The so-called "water intoxication" (hyponatremic shock) may also occur with rapid ingestion of excessive amounts not only of low-mineral water but also tap water. The "intoxication" risk increases with decreasing levels of TDS.
In the past, acute health problems were reported in mountain climbers who had prepared their beverages with melted snow that was not supplemented with necessary ions. A more severe course of such a condition coupled with brain oedema, convulsions and metabolic acidosis was reported in infants whose drinks had been prepared with distilled or low-mineral bottled water (11).
2. Little or no intake of calcium and magnesium from low-mineral water
Calcium and magnesium are both essential elements. Calcium is a substantial component of bones and teeth. In addition, it plays a role in neuromuscular excitability (i.e., decreases it), the proper function of the conducting myocardial system, heart and muscle contractility, intracellular information transmission and the coagulability of blood. Magnesium plays an important role as a cofactor and activator of more than 300 enzymatic reactions including glycolysis, ATP metabolism, transport of elements such as sodium, potassium, and calcium through membranes, synthesis of proteins and nucleic acids, neuromuscular excitability and muscle contraction.
Although drinking water is not the major source of our calcium and magnesium intake, the health significance of supplemental intake of these elements from drinking water may outweigh its nutritional contribution expressed as the proportion of the total daily intake of these elements. Even in industrialized countries, diets deficient in terms of the quantity of calcium and magnesium, may not be able to fully compensate for the absence of calcium and, in particular, magnesium, in drinking water.
For about 50 years, epidemiological studies in many countries all over the world have reported that soft water (i.e., water low in calcium and magnesium) and water low in magnesium is associated with increased morbidity and mortality from cardiovascular disease (CVD) compared to hard water and water high in magnesium. An overview of epidemiological evidence
p 153
is provided by recent review articles (12-15) and summarized in other chapters of this monograph (Calderon and Craun, Monarca et al.). Recent studies also suggest that the intake of soft water, i.e. water low in calcium, may be associated with higher risk of fracture in children (16), certain neurodegenerative diseases (17), pre-term birth and low weight at birth (18) and some types of cancer (19, 20). In addition to an increased risk of sudden death (21-23), the intake of water low in magnesium seems to be associated with a higher risk of motor neuronal disease (24), pregnancy disorders (so-called preeclampsia) (25), and some cancers (26-29).
Specific knowledge about changes in calcium metabolism in a population supplied with desalinated water (i.e., distilled water filtered through limestone) low in TDS and calcium, was obtained from studies carried out in the Soviet city of Shevchenko (3, 30, 31). The local population showed decreased activity of alkaline phosphatase, reduced plasma concentrations of calcium and phosporus and enhanced decalcification of bone tissue. The changes were most marked in women, especially pregnant women and were dependent on the duration of residence in Shevchenko. The importance of water calcium was also confirmed in a one-year study of rats on a fully adequate diet in terms of nutrients and salts and given desalinated water with added dissolved solids of 400 mg/L and either 5 mg/L, 25 mg/L, or 50 mg/L of calcium (3, 32). The animals given water dosed with 5 mg/L of calcium exhibited a reduction in thyroidal and other associated functions compared to the animals given the two higher doses of calcium.
While the effects of most chemicals commonly found in drinking water manifest themselves after long exposure, the effects of calcium and, in particular, those of magnesium on the cardiovascular system are believed to reflect recent exposures. Only a few months exposure may be sufficient consumption time effects from water that is low in magnesium and/or calcium (33).
Illustrative of such short-term exposures are cases in the Czech and Slovak populations who began using reverse osmosis-based systems for final treatment of drinking water at their home taps in 2000-2002. Within several weeks or months various complaints suggestive of acute magnesium (and possibly calcium) deficiency were reported (34). The complaints included cardiovascular disorders, tiredness, weakness or muscular cramps and were essentially the same symptoms listed in the warning of the German Society for Nutrition (7).
3. Low intake of some essential elements and microelements from low-mineral water
Although drinking water, with some rare exceptions, is not the major source of essential elements for humans, its contribution may be important for several reasons. The modern diet of many people may not be an adequate source of minerals and microelements. In the case of borderline deficiency of a given element, even the relatively low intake of the element with drinking water may play a relevant protective role. This is because the elements are usually present in water as free ions and therefore, are more readily absorbed from water compared to food where they are mostly bound to other substances.
Animal studies are also illustrative of the significance of microquantities of some elements present in water. For instance, Kondratyuk (35) reported that a variation in the intake of microelements was associated with up to six-fold differences in their content in muscular tissue.
p 154
given tap water. The haemoglobin differences were even greater when compared with the animalsgiven the mineral supplemented waters.
Lutai (37) conducted a large cohort epidemiological study in the Ust-Ilim region of Russia. The study focused on morbidity and physical development in 7658 adults, 562 children and 1582 pregnant women and their newborns in two areas supplied with water different in TDS. One of these areas was supplied with water lower in minerals (mean values: TDS 134 mg/L, calcium 18.7 mg/L, magnesium 4.9 mg/L, bicarbonates 86.4 mg/L) and the other was supplied with water higher in minerals (mean values: TDS 385 mg/L, calcium 29.5 mg/L, magnesium 8.3 mg/L, bicarbonates 243.7 mg/L). Water levels of sulfate, chloride, sodium, potassium, copper, zinc, manganese and molybdenum were also determined.
The populations of the two areas did not differ from each other in eating habits, air quality, social conditions and time of residence in the respective areas. The population of the area supplied with water lower in minerals showed higher incidence rates of goiter, hypertension, ischemic heart disease, gastric and duodenal ulcers, chronic gastritis, cholecystitis and nephritis. Children living in this area exhibited slower physical development and more growth abnormalities, pregnant women suffered more frequently from edema and anemia. Newborns of this area showed higher morbidity.
The lowest morbidity was associated with water having calcium levels of 30-90 mg/L, magnesium levels of 17-35 mg/L, and TDS of about 400 mg/L (for bicarbonate containing waters). The author concluded that such water could be considered as physiologically optimum.
4. High loss of calcium, magnesium and other essential elements in food prepared in low-mineral water
When used for cooking, soft water was found to cause substantial losses of all essential elements from food (vegetables, meat, cereals). Such losses may reach up to 60 % for magnesium and calcium or even more for some other microelements (e.g., copper 66 %, manganese 70 %, cobalt 86 %). In contrast, when hard water is used for cooking, the loss of these elements is much lower, and in some cases, an even higher calcium content was reported in food as a result of cooking (38-41).
Since most nutrients are ingested with food, the use of low-mineral water for cooking and processing food may cause a marked deficiency in total intake of some essential elements that was much higher than expected with the use of such water for drinking only. The current diet of many persons usually does not provide all necessary elements in sufficient quantities, and therefore, any factor that results in the loss of essential elements and nutrients during the processing and preparation of food could be detrimental for them.
5. Possible increased dietary intake of toxic metals
Increased risk from toxic metals may be posed by low-mineral water in two ways:
1.) higher leaching of metals from materials in contact with water resulting in an increased metal content in drinking water, and
2.) lower protective (antitoxic) capacity of water low in calcium and magnesium.
p 155
Among eight outbreaks of chemical poisoning from drinking water reported in the USA in 1993-1994, there were three cases of lead poisoning in infants who had blood-lead levels of 15 µg/dL, 37 µg/dL, and 42 µg/dL. The level of concern is 10 µg/dL. For all three cases, lead had leached from brass fittings and lead-soldered seams in drinking water storage tanks.
The three water systems used low mineral drinking water that had intensified the leaching process (42). First-draw water samples at the kitchen tap had lead levels of 495 to 1050 µg/L for the two infants with the highest blood lead; 66 µg/L was found in water samples collected at the kitchen tap of the third infant (43).
Calcium and, to a lesser extent, magnesium in water and food are known to have antitoxic activity. They can help prevent the absorption of some toxic elements such as lead and cadmium from the intestine into the blood, either via direct reaction leading to formation of an unabsorbable compound or via competition for binding sites (44-50). Although this protective effect is limited, it should not be dismissed. Populations supplied with low-mineral water may be at a higher risk in terms of adverse effects from exposure to toxic substances compared to populations supplied with water of average mineralization and hardness.
6. Possible bacterial contamination of low-mineral water
All water is prone to bacterial contamination in the absence of a disinfectant residual either at source or as a result of microbial re-growth in the pipe system after treatment. Re-growth may also occur in desalinated water. Bacterial re-growth within the pipe system is encouraged by higher initial temperatures, higher temperatures of water in the distribution system due to hot climates, lack of a residual disinfectant, and possibly greater availability of some nutrients due to the aggressive nature of the water to materials in contact with it.
Although an intact desalination membrane should remove all bacteria, it may not be 100 % effective (perhaps due to leaks) as can be documented by an outbreak of typhoid fever caused by reverse osmosis-treated water in Saudi Arabia in 1992 (51).
Thus, virtually all waters including desalinated waters are disinfected after treatment. Non pathogenic bacterial re-growth in water treated with different types of home water treatment devices was reported by Geldreich et al. (52) and Payment et al. (53, 54) and many others.
The Czech National Institute of Public Health (34) in Prague has tested products intended for contact with drinking water and found, for example, that the pressure tanks of reverse osmosis units are prone to bacterial regrowth, primarily do to removal of residual disinfectant by the treatment. They also contain a rubber bag whose surface appears to be favourable for bacterial growth.
III. DESIRABLE MINERAL CONTENT OF DEMINERALISED DRINKING WATER
The corrosive nature of demineralised water and potential health risks related to the distribution and consumption of low TDS water has led to recommendations of the minimum and optimum mineral content in drinking water and then, in some countries, to the establishment of obligatory values in the respective legislative or technical regulations for drinking water quality. Organoleptic characteristics and thirst-quenching capacity were also considered in the recommendations. For example, human volunteer studies (3) showed that the water temperatures of 15-350 C best satisfied physiological needs. Water temperatures above 350 or below 150 C
p 156
resulted in a reduction in water consumption. Water with a TDS of 25-50 mg/L was described tasteless (3).
1. The 1980 WHO report
The recommendations were based on extensive experimental studies conducted in rats, dogs and human volunteers. Water exposures included Moscow tap water, desalinated water of approximately 10 mg/L TDS, and laboratory-prepared water of 50, 100, 250, 300, 500, 750, 1000, and 1500 mg/L TDS using the following constituents and proportions: Cl-(40%), HCO 3 (32%), SO4(28%) / Na (50%), Ca (38%), Mg (12%).
A number of health outcomes were investigated including: dynamics of body weight, basal and nitrogen metabolism, enzyme activity, water-salt homeostasis and its regulatory system, mineral content of body tissues and fluids, hematocrit, and ADH activity. The optimal TDS was associated with the lowest incidence of adverse effect, negative changes to the human, dog, or rat, good organoleptic characteristics and thirst-quenching properties, and reduced corrosivity of water.
In addition to the TDS levels, the report (3) recommended that the minimum calcium content of desalinated drinking water should be 30 mg/L. These levels were based on health
concerns with the most critical effects being hormonal changes in calcium and phosphorus metabolism and reduced mineral saturation of bone tissue. Also, when calcium is increased to 30 mg/L, the corrosive activity of desalinated water would be appreciably reduced and the water would be more stable (3).
concerns with the most critical effects being hormonal changes in calcium and phosphorus metabolism and reduced mineral saturation of bone tissue. Also, when calcium is increased to 30 mg/L, the corrosive activity of desalinated water would be appreciably reduced and the water would be more stable (3).
The report (3) also recommended a bicarbonate ion content of 30 mg/L as a minimum essential level needed to achieve acceptable organoleptic characteristics, reduced corrosivity, and an equilibrium concentration for the recommended minimum level of calcium.
2. Recent recommendations
The water in city A water had the lowest levels of calcium and magnesium (3.0 mg/L calcium and 2.4 mg/L magnesium). The water in city B had slightly higher levels (18.0 mg/L calcium and 5.0 mg/L magnesium). The highest levels were in city C (22.0 mg/L calcium and 11.3 mg/L magnesium) and city D (45.0 mg/L calcium and 26.2 mg/L magnesium).
Women living in cities A and B more frequently showed cardiovascular changes (as measured by ECG), higher blood pressure, somatoform autonomic dysfunctions, headache, dizziness, and osteoporosis (as measured by X-ray absorptiometry) compared to those of cities C and D. These results suggest that the minimum magnesium content of drinking water should be 10 mg/L and the minimum calcium content should be 20 mg/L rather than 30 mg/L as recommended in the 1980 WHO report (3).
Based on the currently available data, various researchers have recommended that the following levels of calcium, magnesium, and water hardness should be in drinking water:
• For magnesium, a minimum of 10 mg/L (33, 56) and an optimum of about 20-30 mg/L (49, 57);
p 157
• For calcium, a minimum of 20 mg/L (56) and an optimum of about 50 (40-80) mg/L (57, 58);
• For total water hardness, the sum of calcium and magnesium should be 2 to 4 mmol/L (37, 50, 59, 60).
At these concentrations, minimum or no adverse health effects were observed. The maximum protective or beneficial health effects of drinking water appeared to occur at the
estimated desirable or optimum concentrations. The recommended magnesium levels were based on cardiovascular system effects, while changes in calcium metabolism and ossification were used as a basis for the recommended calcium levels. The upper limit of the hardness optimal range was derived from data that showed a higher risk of gall stones, kidney stones, urinary stones, arthrosis and arthropathies in populations supplied with water of hardness higher than 5 mmol/L. Long-term intake of drinking water was taken into account in estimating these concentrations. For short-term therapeutic indications of some waters, higher concentrations of these elements may be considered.
V. GUIDELINES AND DIRECTIVES FOR CALCIUM, MAGNESIUM, AND HARDNESS LEVELS IN DRINKING WATER
The WHO in the 2nd edition of Guidelines for Drinking-water Quality (61) evaluated calcium and magnesium in terms of water hardness but did not recommend either minimum levels or maximum limits for calcium, magnesium, or hardness.The first European Directive (62) established a requirement for minimum hardness for softened or desalinated water (≥ 60 mg/L as calcium or equivalent cations). This requirement appeared obligatorily in the national legislations of all EEC members, but this Directive expired in December 2003 when a new Directive (63) became effective. The new Directive does not contain a requirement for calcium, magnesium, or water hardness levels. On the other hand, it does not prevent member states from implementing such a requirement into their national legislation.
Only a few EU Member States (e.g. the Netherlands) have included calcium, magnesium, or water hardness into their national regulations as a binding requirement. Some EU Member States (e.g. Austria, Germany) included these parameters at lower levels as unbinding regulations, such as technical standards (e.g., different measures for reduction of water corrosivity). All four Central European countries that became part of the EU in May 2004 have included the following requirements in their respective regulations but varying in binding power;
• Czech Republic (2004): for softened water ≥ 30 mg/L calcium and ≥ 10 mg/L magnesium; guideline levels of 40-80 mg/L calcium and 20–30 mg/L magnesium (hardness as Σ Ca + Mg = 2.0 – 3.5 mmol/L).
• Hungary (2001): hardness 50 – 350 mg/L (as CaO); minimum required concentration of 50
mg/L must be met in bottled drinking water, new water sources, and softened and desalinated water.
• Poland (2000): hardness 60–500 mg/L (as CaCO3).
• Slovakia (2002): guideline levels > 30 mg/L calcium and 10 – 30 mg/L magnesium.
The Russian technical standard Astronaut environment in piloted spaceships – general medical and technical requirements (64) defines qualitative requirements for recycled water intended for drinking in spaceships. Among other requirements, the TDS should range between 100 and 1000 mg/L with minimum levels of fluoride, calcium and magnesium being specified by
p 158
• Hungary (2001): hardness 50 – 350 mg/L (as CaO); minimum required concentration of 50
mg/L must be met in bottled drinking water, new water sources, and softened and desalinated water.
• Poland (2000): hardness 60–500 mg/L (as CaCO3).
• Slovakia (2002): guideline levels > 30 mg/L calcium and 10 – 30 mg/L magnesium.
The Russian technical standard Astronaut environment in piloted spaceships – general medical and technical requirements (64) defines qualitative requirements for recycled water intended for drinking in spaceships. Among other requirements, the TDS should range between 100 and 1000 mg/L with minimum levels of fluoride, calcium and magnesium being specified by
p 158
a special commission separately for each cosmic flight. The focus is on how to supplement recycled water with a mineral concentrate to make it “physiologically valuable” (65).
V. CONCLUSIONS
Drinking water should contain minimum levels of certain essential minerals (and other components such as carbonates). Unfortunately, over the two past decades, little research attention has been given to the beneficial or protective effects of drinking water substances. The main focus has been on the toxicological properties of contaminants. Nevertheless, some studies have attempted to define the minimum content of essential elements or TDS in drinking water, and some countries have included requirements or guidelines for selected substances in their drinking water regulations. The issue is relevant not only where drinking water is obtained by desalination (if not adequately re-mineralised) but also where home treatment or central water treatment reduces the content of important minerals and low-mineral bottled water is consumed.
Drinking water manufactured by desalination is stabilized with some minerals, but this is usually not the case for water demineralised as a result of household treatment. Even when
stabilized, the final composition of some waters may not be adequate in terms of providing health benefits. Although desalinated waters are supplemented mainly with calcium (lime) or other carbonates, they may be deficient in magnesium and other microelements such as fluorides and potassium. Furthermore, the quantity of calcium that is supplemented is based on technical considerations (i.e., reducing the aggressiveness) rather than on health concerns. Possibly none of the commonly used ways of re-mineralization could be considered optimum, since the water does not contain all of its beneficial components. Current methods of stabilization are primarily intended to decrease the corrosive effects of demineralised water.
Demineralised water that has not been remineralized, or low-mineral content water – in the light of the absence or substantial lack of essential minerals in it – is not considered ideal drinking water, and therefore, its regular consumption may not be providing adequate levels of some beneficial nutrients. This chapter provides a rationale for this conclusion. The evidence in terms of experimental effects and findings in human volunteers related to highly demineralised water is mostly found in older studies, some of which may not meet current methodological criteria. However, these findings and conclusions should not be dismissed. Some of these studies were unique, and the intervention studies, although undirected, would hardly be scientifically, financially, or ethically feasible to the same extent today. The methods, however, are not so questionable as to necessarily invalidate their results. The older animal and clinical studies on health risks from drinking demineralised or low-mineral water yielded consistent results both with each other, and recent research has tended to be supportive.
Sufficient evidence is now available to confirm the health consequences from drinking water deficient in calcium or magnesium. Many studies show that higher water magnesium is related to decreased risks for CVD and especially for sudden death from CVD. This relationship has been independently described in epidemiological studies with different study designs, performed in different areas, different populations, and at different times. The consistent epidemiological observations are supported by the data from autopsy, clinical, and animal studies. Biological plausibility for a protective effect of magnesium is substantial, but the specificity is less evident due to the multifactorial aetiology of CVD. In addition to an increased risk of sudden death, it has been suggested that intake of water low in magnesium may be associated with a higher risk of motor neuronal disease, pregnancy disorders (so-called preeclampsia), sudden death in infants, and some types of cancer. Recent studies suggest that the intake of soft water, i.e. water low in calcium, is associated with a higher risk of fracture in children, certain neurodegenerative
p 159
diseases, pre-term birth and low weight at birth and some types of cancer. Furthermore, the possible role of water calcium in the development of CVD cannot be excluded.
International and national authorities responsible for drinking water quality should consider guidelines for desalination water treatment, specifying the minimum content of the relevant elements such as calcium and magnesium and TDS. If additional research is required to establish guidelines, authorities should promote targeted research in this field to elaborate the health benefits. If guidelines are established for substances that should be in deminerialised water, authorities should ensure that the guidelines also apply to uses of certain home treatment devices and bottled waters.
Health Risk from Drinking Demineralized Water (PDF Download Available). Available from: https://www.researchgate.net/publication/252043662_Health_Risk_from_Drinking_Demineralized_Water [accessed Sep 28, 2017].
References:
For references referred to in the above article, please go to the above external links.
Labels:
Brain Health,
Calcium,
Cardiovascular Health,
Distilled Water,
Heart health,
Heavy Metals,
Magnesium,
MUST READ,
Reverse Osmosis,
Water,
Water Filtration,
WHO
Tuesday, 26 September 2017
Neck massage by barber may damage nerve, cause paralysis
NEW DELHI: A haircut at most barber shops in India is followed by a head massage. It often ends with a 'neck-crack', when the barber holds you by the chin and tilts the neck sharply to the left and right.
Durgesh Nandan Jha| TNN | Updated: Sep 19, 2017, 18:33 IST

Like countless Indian men, Ajay Kumar, 54, came away from the salon feeling refreshed after the haircut-and-massage routine last month. Soon, however, he became increasingly breathless.
It turned out that the neck crack had damaged his phrenic nerves that control the diaphragm, which in turn controls breathing. Kumar, a PSU employee, had to be put on mechanical ventilation. "He has been put on non-invasive ventilation for breathing support and may continue to be on it," said Dr Anand Jaiswal, director of respiratory and sleep medicine at Medanta - The Medicity.
Jaiswal said Kumar's diaphragm was paralysed and he may need ventilator support throughout his life because the nerve rarely regenerates spontaneously.
The doctor also cautioned people against getting a neck massage at saloons.
"The neck massage and neck-crack that barbers ritually perform after a haircut can cause long-term damage to neck joints and surrounding tissues, muscles or nerves or even cause bilateral diaphragmatic paralysis like in this case," the doctor said.
Breathlessness is often associated with malfunctioning of the heart or lung. When Kumar came to Medanta with the problem about a month back, doctors said they conducted a series of tests to diagnose the problem while treating him for other suspected causes. When the problem persisted, they looked at medical literature for clues. "While examining him, we noticed Kumar had a paradoxical breathing pattern. His chest was moving inward instead of expanding. This abnormal chest movement affected the breathing pattern and led to a drop in his blood oxygen levels," Jaiswal explained.
He added, "As we investigated the possible reasons for the paradoxical breathing, a neurological examination revealed Kumar was suffering from a damaged phrenic nerve. Because he had no other existing illness to explain it, and literature had some examples about neck-crack causing such problem, we questioned Kumar and found that his neck massage had caused the condition."
In a typical Indian barber shop, it is not uncommon to find customers such as Kumar who like to end their haircut with a massage and a customary neck-crack. People get it done because they find it relaxing and believe a neck massage is a good practice to keep the neck loose. Dr Shakir Husain, director, stroke and neurovascular clinic at Neo hospital, said these neck massages can also lead to dissection of the vertebral artery.
"If the dissection or tear is minor, it heals naturally. Blood thinners have to be given for two to three months. But in some cases, surgery has to be done to clear the aneurysm caused due to the tear," he said. Damage to the artery during neck manipulation is also seen in patients opting for chiropractic therapy for musculoskeletal problems. "It may lead to disabling stroke or fatality," the senior neurosurgeon said.
http://timesofindia.indiatimes.com/city/delhi/neck-massage-by-barber-may-damage-nerve-cause-paralysis/articleshow/60739804.cms
Durgesh Nandan Jha| TNN | Updated: Sep 19, 2017, 18:33 IST

Like countless Indian men, Ajay Kumar, 54, came away from the salon feeling refreshed after the haircut-and-massage routine last month. Soon, however, he became increasingly breathless.
It turned out that the neck crack had damaged his phrenic nerves that control the diaphragm, which in turn controls breathing. Kumar, a PSU employee, had to be put on mechanical ventilation. "He has been put on non-invasive ventilation for breathing support and may continue to be on it," said Dr Anand Jaiswal, director of respiratory and sleep medicine at Medanta - The Medicity.
Jaiswal said Kumar's diaphragm was paralysed and he may need ventilator support throughout his life because the nerve rarely regenerates spontaneously.
The doctor also cautioned people against getting a neck massage at saloons.
"The neck massage and neck-crack that barbers ritually perform after a haircut can cause long-term damage to neck joints and surrounding tissues, muscles or nerves or even cause bilateral diaphragmatic paralysis like in this case," the doctor said.
Breathlessness is often associated with malfunctioning of the heart or lung. When Kumar came to Medanta with the problem about a month back, doctors said they conducted a series of tests to diagnose the problem while treating him for other suspected causes. When the problem persisted, they looked at medical literature for clues. "While examining him, we noticed Kumar had a paradoxical breathing pattern. His chest was moving inward instead of expanding. This abnormal chest movement affected the breathing pattern and led to a drop in his blood oxygen levels," Jaiswal explained.
He added, "As we investigated the possible reasons for the paradoxical breathing, a neurological examination revealed Kumar was suffering from a damaged phrenic nerve. Because he had no other existing illness to explain it, and literature had some examples about neck-crack causing such problem, we questioned Kumar and found that his neck massage had caused the condition."
In a typical Indian barber shop, it is not uncommon to find customers such as Kumar who like to end their haircut with a massage and a customary neck-crack. People get it done because they find it relaxing and believe a neck massage is a good practice to keep the neck loose. Dr Shakir Husain, director, stroke and neurovascular clinic at Neo hospital, said these neck massages can also lead to dissection of the vertebral artery.
"If the dissection or tear is minor, it heals naturally. Blood thinners have to be given for two to three months. But in some cases, surgery has to be done to clear the aneurysm caused due to the tear," he said. Damage to the artery during neck manipulation is also seen in patients opting for chiropractic therapy for musculoskeletal problems. "It may lead to disabling stroke or fatality," the senior neurosurgeon said.
http://timesofindia.indiatimes.com/city/delhi/neck-massage-by-barber-may-damage-nerve-cause-paralysis/articleshow/60739804.cms
The Dangers of Drinking Reverse Osmosis Water
Scientist and researchers worldwide have repeatedly demonstrated that long-term consumption of reverse osmosis water (RO) water is bad for your health.
June 7, 2014
 Just about, everyone knows that Reverse Osmosis (RO) systems excel at removing water impurities, similar to distilled water they both take out the natural minerals in the water Asia and the European countries call it dead water, in some countries it is even banned, few are aware that they also remove the beneficial minerals. In fact, the reverse osmosis process removes 92-99% of beneficial calcium and magnesium. What is the big deal?
Just about, everyone knows that Reverse Osmosis (RO) systems excel at removing water impurities, similar to distilled water they both take out the natural minerals in the water Asia and the European countries call it dead water, in some countries it is even banned, few are aware that they also remove the beneficial minerals. In fact, the reverse osmosis process removes 92-99% of beneficial calcium and magnesium. What is the big deal?
“Disease and early death is more likely to be seen with the long term drinking of distilled water, reverse osmosis water”. Zoltan P. Roma MD MSc, In other words reverse osmosis can cause death.
After analyzing hundreds of scientific studies concerning deminerialized or reverse osmosis water, the World Health Organization released a report stating that such water “has a definite adverse influence on the animal and human organism.”
Consumers have been so concerned with removing as many things from water as possible that they have forgotten to ask if the resulting water actually improves health or causes health problems. It is assumed that no toxins equals better health, but there is simply more to healthful water than a lack of toxins, as the World Health Organization clearly points out.
What is alarming is that consuming reverse osmosis water for even just a few months can create serious side effects. “The effects of most chemicals commonly found in drinking water manifest themselves after long exposure.” However “only a few months exposure may be sufficient ‘consumption time effects’ from water that is low in magnesium and/or calcium.
Illustrative of such short-term exposures are cases in the Czech and Slovak populations who began using reverse osmosis-based systems for final treatment of drinking water at their home taps in 2000-2002. Within several weeks or months various health complaints suggestive of acute magnesium (and possibly calcium) deficiency were reported.Among these complaints were cardiovascular disorders, tiredness, weakness or muscular cramps.” Again, serious side effects within just several weeks or months.
However, it gets even worse. Because reverse osmosis water does not have enough minerals, when it is consumed, it also leaches minerals from the body. This means that the minerals being consumed in food and vitamins are being urinated away. Less minerals consumed plus more minerals, being excreted equals serious negative side effects and big health problems. In a scientific study performed to see if minerals consumed in food can make up for the lack of minerals in reverse osmosis water, scientists concluded that “reduced mineral intake from water was not compensated by their diets…low-mineral water was responsible for an increased elimination of minerals from the body.”
“It has been adequately demonstrated that consuming water of low mineral content has a negative effect on homeostasis mechanisms, compromising the mineral and water metabolism in the body.” Consumption of reverse osmosis water “leads to the dilution of the electrolytes dissolved in the body water. Inadequate body water redistribution between compartments may compromise the function of vital organs. Side effects at the very beginning of this condition include tiredness, weakness and headache; more severe symptoms are muscular cramps and impaired heart rate.”
What about those reverse osmosis systems that add minerals back in?
One popular trend since the negative side effects of consuming reverse osmosis water emerged is the practice of adding minerals back into the reverse osmosis water. On this trend, the World Health Organization states that “possibly none of the commonly used ways of re-mineralization could be considered optimum, since the water does not contain all of its beneficial components. In the case of borderline deficiency of a given element, even the relatively low intake of the element with drinking water may play a relevant protective role.” It is practically impossible to recreate natural water with all of its minerals and trace elements from reverse osmosis treated water. Why risk the side effects of reverse osmosis water in any form if healthful alternatives are available?
Since the early 1960’s, epidemiological studies in many countries all over the world have reported that water low in calcium and magnesium is associated with increased morbidity and mortality from cardiovascular disease.
When used for cooking, reverse osmosis water was found to cause substantial losses of all essential elements from food (vegetables, meat, cereals). Such losses may reach up to 60 % for magnesium and calcium or even more for some other microelements (e.g., copper 66 %, manganese 70 %, cobalt 86 %). In contrast, when mineralized water is used for cooking, the loss of these elements is much lower, and in some cases, even higher calcium content was reported in food because of cooking.
In a multi-city study, women living in cities with low-mineral water more frequently showed cardiovascular changes (as measured by ECG), higher blood pressure, somatoform autonomic dysfunctions, headache, dizziness, and osteoporosis (as measured by X-ray absorptiometry) compared to those of cities with higher mineral content water.
The second website that drives a dagger into the RO industry myth that its pure water is healthy comes from the World Health Organization (W.H.O.). The WHO provides us with a Position Paper titled “The Health risks from drinking demineralized water” which was written by F. Kozisek. You can tell by the title where the article is headed.
Here is a link to the article:
The final report, published as an internal working document (WHO 1980), concluded that “not only does completely demineralised water (distillate) have unsatisfactory organoleptic properities, but it also has a definite adverse influence on the animal and human organism.”
Scientific testing and the best “unbiased” brains in the world have repeatedly demonstrated that long term consumption of demineralized (RO) water is bad for your health.
https://drinknatureswater.wordpress.com/2014/06/07/the-dangers-of-drinking-reverse-osmosis-water/
For the full WHO report read:
1. WHO report: Health Risks from Drinking Demineralised Water - MUST READ
2. Evaluating household water treatment options
- - - - - - - - - - - - - -
The following article is written by the International Water Association - it explains what reverse osmosis is. Contrary to the findings of the WHO report, it also says "Consumers should not be concerned about the removal of minerals by RO system". Here it is, for your consumption:
Reverse Osmosis will generally remove salt, manganese, iron, flouride, lead, and calcium (Binnie et. al., 2002). Most mineral constituents of water are physically larger than water molecules and they are trapped by the semi-permeable membrane and removed from drinking water when filtered through a RO (AllAboutWater.org, 2004). Meanwhile, consumers are concerned about the removal of minerals from their drinking water.
Reverse Osmosis Remove Minerals
Reverse Osmosis (RO) removed more than 90-99.99% of all the contaminants including minerals from the drinking water supply (see Figure 1). RO removes minerals because they have larger molecules than water. The subject of minerals and RO created controversy and disagreement among water and health professionals. The World Health Organization (WHO) made clarification that majority of healthy minerals are needed for human body is from food or dietary supplementary sources and not from drinking tap water. In addition, minerals found in water can be harmful to human health. The evidence is strong that calcium and magnesium are essential elements for human body (WQA, 2011).
However, its a weak argument to suggest that we should make up this deficiency through water consumption (WQA, 2011). Tap water presents a variety of inorganic minerals which human body has difficulty absorbing (Misner, 2004). Their presence is suspect in a wide array of degenerative diseases, such as hardening of the arteries, arthritis, kidney stones, gall stones, glaucoma, cataracts, hearing loss, emphysema, diabetes, and obesity. What minerals are available, especially in "hard" tap water, are poorly absorbed, or rejected by cellular tissue sites, and, if not evacuated, their presence may cause arterial obstruction, and internal damage (Dennison, 193; Muehling, 1994; Banik, 1989).
However, its a weak argument to suggest that we should make up this deficiency through water consumption (WQA, 2011). Tap water presents a variety of inorganic minerals which human body has difficulty absorbing (Misner, 2004). Their presence is suspect in a wide array of degenerative diseases, such as hardening of the arteries, arthritis, kidney stones, gall stones, glaucoma, cataracts, hearing loss, emphysema, diabetes, and obesity. What minerals are available, especially in "hard" tap water, are poorly absorbed, or rejected by cellular tissue sites, and, if not evacuated, their presence may cause arterial obstruction, and internal damage (Dennison, 193; Muehling, 1994; Banik, 1989).
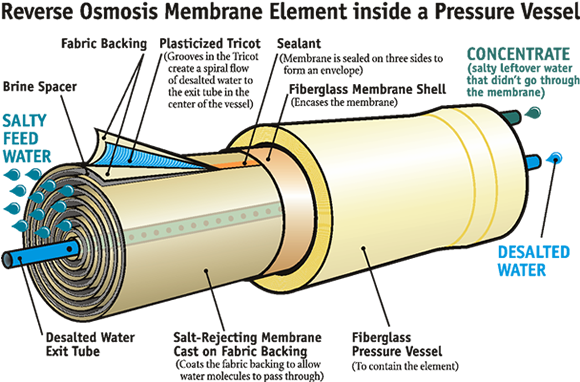
Figure 1. Reverse Osmosis Membrane (Source:DOI-BUR, 2009)
Organic Minerals vs. Inorganic Minerals
There are two types of minerals in water, organic and inorganic. Human physiology has a biological affinity for organic minerals. Most organic minerals for our body functions come from dietary plant foods (Misner, 2004). A growing plant converts the inorganic minerals from the soils to a useful organic mineral (Misner, 2004). When an organic mineral (from a plant food) enters the stomach it must attach itself to a specific protein-molecule (chelation) in order to be absorbed, then it gains access to the tissue sites where it is needed (Misner, 2004). Once a plant mineral is divested within the body, it is utilized as a coenzyme for composing body fluids, forming blood and bone cells, and the maintaining of healthy nerve transmission (Balch & Balch 1990).
Reverse Osmosis has Little Affect on Water pH
Water pH levels will automatically change when it is ingested and comes into contact with the food in your stomach (Wise, 2011). Even on an empty stomach, your stomach acid alone is already several times more acidic than RO water (pH 6-8) with a pH level of 2 (Wise, 2011). The human body regulates pH levels constantly to find balance and equilibrium (see Figure 2). Therefore under normal conditions it will always maintain a neutral 7.4 pH balance (Wise, 2011).
The healthy body is very robust and it will restore homeostatic pH fairly quickly and easily (Wise 2011). Soft drinks and sports drinks typically have a pH level of 2.5, orange juice has a 3 pH and coffee has a 4 pH level and we drink these beverages all the time without problems (Wise, 2011).
The healthy body is very robust and it will restore homeostatic pH fairly quickly and easily (Wise 2011). Soft drinks and sports drinks typically have a pH level of 2.5, orange juice has a 3 pH and coffee has a 4 pH level and we drink these beverages all the time without problems (Wise, 2011).
Figure 2. Comparison of pH Levels (Source: Wise, 2011)
Conclusion
Water filtered or treated by RO is pure, clean, and healthy. RO treatment system is currently the only technology that can remove most of the emerging contaminants (i.e., prescription drugs and perchlorate) including other contaminants (i.e., Arsenic, Cyanide, and Fluoride) that are difficult to remove by other treatment methods. No more ingesting of harmful inorganic minerals means the body will no longer be stressed with trying to absorb something that wasn't supposed to be there in the first place (Wise, 2011). Consumers should not be concerned about the removal of minerals by RO system. WHO (2009) and WQA (2011) pointed out, that the human body obtains vast majority of minerals from food or supplements, not from drinking water.
https://www.iwapublishing.com/news/reverse-osmosis-and-removal-minerals-drinking-water
Note: The above article is by the International Water Association.
Note: The above article is by the International Water Association.
- - - - - -
Labels:
Calcium,
Cardiovascular Health,
Magnesium,
Reverse Osmosis,
Water,
Water Filtration,
WHO
Subscribe to:
Comments (Atom)