Swiss company Roche Diagnostics claims that it has created a test with 100% accuracy and could provide hundreds of thousands to the NHS every week.
4 May 2020
 |
| Current testing only identifies those who have it now |
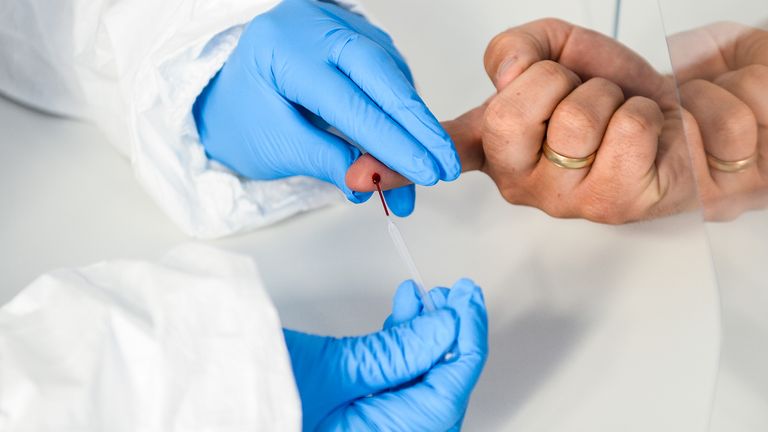
Roche claims its lab-based ‘Elecsys’ test can spot 100% of people who have had the virus with no ‘false negatives’ at all.
Roche Diagnostics said it is already ‘in dialogue’ with the NHS and the UK Government about a ‘phased roll-out of the test from mid-May’.
A spokesman added: ‘We will be able to provide hundreds of thousands of antibody tests to the UK per week.
‘Hospitals and reference laboratories can run the test on fully-automated equipment already widely installed by Roche Diagnostics at sites across the UK with results provided in 18 minutes.’
Another UK research firm claims it has also developed a ‘fast and accurate’ coronavirus antibody test – but it fears the NHS could miss out amid interest in Europe for the tests.
Researchers for blood-screening company Quotient, based in Edinburgh, have developed a new test for whether people are immune to Covid-19 by spotting whether a person has developed antibodies to the disease.
The tests involve using serological screening machines – each has capacity for up to 3,000 tests a day and produces results in 35 minutes with 99.8% accuracy, scientists said.
The company says it has 12 screening machines available, with a further 20 expected to be ready by the end of the year, but it has already had talks with interested parties across Europe.
Quotient is calling for the UK and Scottish governments to begin talks so that the NHS might be able to benefit.
While the UK Government says it has laboratory capability to test for coronavirus immunity, it is currently being used for survey testing of existing blood samples and the capacity is not known.
It is also attempting to develop home testing kits, rather than requiring analysis in laboratories, but so far these have proved unreliable.
On Friday, Quotient received European regulatory approval for the MosaiQ serological screening machines with 100% sensitivity and 99.8% specificity, meaning there is a low chance of a misread or ‘false positive’.
Chief executive Franz Walt – who was managing director of a laboratory that developed the first diagnostic test for Sars in 2003 – said: ‘We are truly proud to have developed such a fast and accurate test.
‘This is an outstanding performance by our teams in both Edinburgh and Switzerland.
‘We now want to make sure that we can help as many people as possible as quickly as possible. ‘We have strong roots in the UK and want to speak to ministers there so MosaiQ can be used in the amazing national effort to tackle Coronavirus and relaunch the economy.
‘We realise ministers and the NHS are incredibly busy but are keen to talk given the strong interest from across Europe in the product.’
Ed Farrell, chief operating officer at the Edinburgh office, added: ‘We’re incredibly proud of all our work here in Scotland and Switzerland.
‘100% accurate’ antibody tests ‘could be rolled out within 2 weeks’
ALSO:
SEE:
https://healthticket.blogspot.com/2020/05/roche-and-quotient-antibody-testing-in.html
https://healthticket.blogspot.com/2020/05/new-100-accurate-covid-19-antibody-test.html
https://healthticket.blogspot.com/2020/05/100-accurate-antibody-tests-could-be.html